Co On The Periodic Table
Crystal Structure of Cobalt
The solid state structure of Cobalt is Uncomplicated Hexagonal.
The Crystal structure can be described in terms of its unit Jail cell. The unit Cells repeats itself in 3 dimensional space to form the structure.
Unit Cell Parameters
The unit cell is represented in terms of its lattice parameters, which are the lengths of the cell edges Lattice Constants (a, b and c)
| a | b | c |
|---|---|---|
| 250.71 pm | 250.71 pm | 406.95 pm |
and the angles betwixt them Lattice Angles (alpha, beta and gamma).
| alpha | beta | gamma |
|---|---|---|
| π/2 | π/2 | 2 π/three |
The positions of the atoms inside the unit cell are described by the set of atomic positions ( teni, yi, zi) measured from a reference lattice betoken.
The symmetry properties of the crystal are described by the concept of space groups. All possible symmetric arrangements of particles in iii-dimensional space are described by the 230 infinite groups (219 distinct types, or 230 if chiral copies are considered distinct.
Cobalt Atomic and Orbital Backdrop
Cobalt atoms have 27 electrons and the electronic shell structure is [ 2, eight, 15, 2 ] with Atomic Term Symbol (Quantum Numbers) fourF9/2.
| Atomic Number | 27 |
| Number of Electrons (with no charge) | 27 |
| Number of Protons | 27 |
| Mass Number | 59 |
| Number of Neutrons | 32 |
| Beat construction (Electrons per energy level) | 2, 8, 15, 2 |
| Electron Configuration | [Ar] 3d7 4s2 |
| Valence Electrons | 3d7 4s2 |
| Valence (Valency) | 4 |
| Main Oxidation States | 2, three |
| Oxidation States | -iii, -1, 0, 1, 2, iii, iv, v |
| Atomic Term Symbol (Quantum Numbers) | 4Fnine/two |
Bohr Diminutive Model of Cobalt - Electrons per energy level

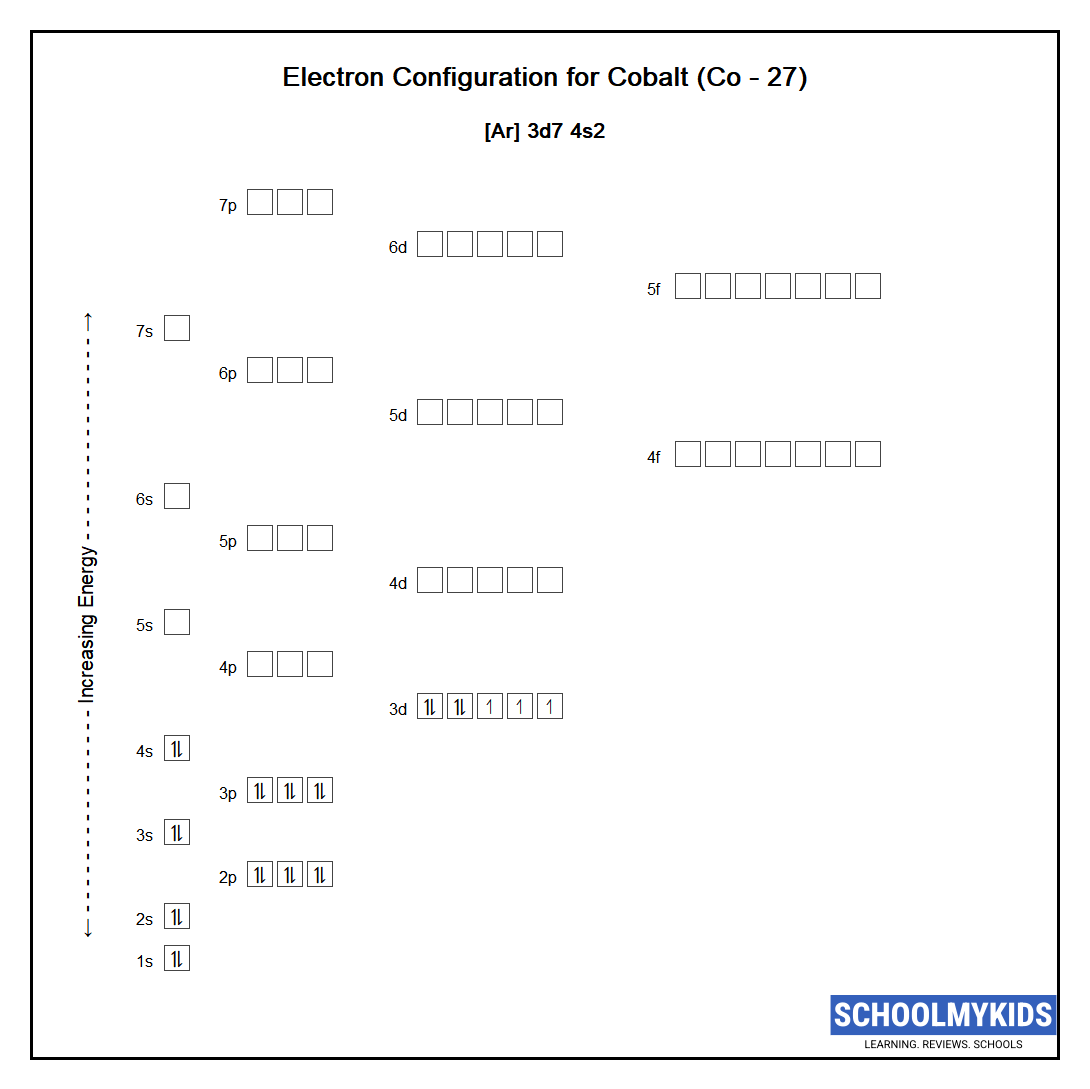
Footing State Electronic Configuration of Cobalt - neutral Cobalt atom
Abbreviated electronic configuration of Cobalt
The ground land abbreviated electronic configuration of Neutral Cobalt atom is [Ar] 3d7 4s2 . The portion of Cobalt configuration that is equivalent to the noble gas of the preceding period, is abbreviated as [Ar] . For atoms with many electrons, this notation can go lengthy and and then an abbreviated annotation is used. This is important as information technology is the Valence electrons 3d7 4s2 , electrons in the outermost shell that determine the chemical properties of the element.
Unabbreviated electronic configuration of neutral Cobalt
Complete footing state electronic configuration for the Cobalt atom, Unabbreviated electronic configuration
1s2 2s2 2p6 3s2 3p6 3d7 4s2
Electrons are filled in diminutive orbitals as per the guild adamant by the Aufbau principle, Pauli Exclusion Principle and Hund'southward Rule.
Diminutive Construction of Cobalt
Cobalt diminutive radius is 152 pm , while it's covalent radius is 126 pm .
| Atomic Radius Calculated | 152 pm ( i.52 Å) |
| Diminutive Radius Empirical | 135 pm ( 1.35 Å) |
| Atomic Volume | 6.62 cm3/mol |
| Covalent Radius | 126 pm ( 1.26 Å) |
| Van der Waals Radius | - |
| Neutron Cross Section | 37.2 |
| Neutron Mass Absorption | 0.021 |
Atomic Spectrum of Cobalt

Cobalt Chemical Properties: Cobalt Ionization Energies and electron affinity
The electron affinity of Cobalt is 63.7 kJ/mol .
Ionization Energy of Cobalt
Refer to tabular array below for Ionization energies of Cobalt
| Ionization energy number | Enthalpy - kJ/mol |
|---|---|
| 1st | 760.4 |
| 2nd | 1648 |
| 3rd | 3232 |
| 4th | 4950 |
| 5th | 7670 |
| 6th | 9840 |
| seventh | 12440 |
| 8th | 15230 |
| ninth | 17959 |
| tenth | 26570 |
| 11th | 29400 |
| 12th | 32400 |
| 13th | 36600 |
| 14th | 39700 |
| 15th | 42800 |
| 16th | 49396 |
| 17th | 52737 |
| 18th | 134810 |
| 19th | 145170 |
| 20th | 154700 |
| 21st | 167400 |
| 22nd | 178100 |
| 23rd | 189300 |
| 24th | 204500 |
| 25th | 214100 |
| 26th | 920870 |
| 27th | 966023 |
Cobalt Physical Properties
Refer to below tabular array for Cobalt Concrete Backdrop
| Density | 8.9 g/cm3 (when liquid at k.p density is $ vii.75 g/cm3 ) |
| Tooth Volume | 6.62 cm3/mol |
Elastic Properties
Hardness of Cobalt - Tests to Measure of Hardness of Element
Cobalt Electrical Properties
Cobalt is Conductor of electricity. Refer to table below for the Electric properties of Cobalt
Cobalt Heat and Conduction Backdrop
Cobalt Magnetic Properties
Optical Properties of Cobalt
Audio-visual Backdrop of Cobalt
Cobalt Thermal Backdrop - Enthalpies and thermodynamics
Refer to table beneath for Thermal properties of Cobalt
Enthalpies of Cobalt
Cobalt Isotopes - Nuclear Properties of Cobalt
Cobalt has 29 isotopes, with between 47 and 75 nucleons. Cobalt has ane stable naturally occuring isotopes.
Isotopes of Cobalt - Naturally occurring stable Isotopes: 59Co .
| Isotope | Z | N | Isotope Mass | % Abundance | T half | Decay Mode |
|---|---|---|---|---|---|---|
| 47Co | 27 | 20 | 47 | Synthetic | ||
| 48Co | 27 | 21 | 48 | Constructed | ||
| 49Co | 27 | 22 | 49 | Constructed | ||
| 50Co | 27 | 23 | 50 | Synthetic | ||
| 51Co | 27 | 24 | 51 | Synthetic | ||
| 52Co | 27 | 25 | 52 | Constructed | ||
| 53Co | 27 | 26 | 53 | Synthetic | ||
| 54Co | 27 | 27 | 54 | Synthetic | ||
| 55Co | 27 | 28 | 55 | Synthetic | ||
| 56Co | 27 | 29 | 56 | Synthetic | ||
| 57Co | 27 | 30 | 57 | Synthetic | ||
| 58Co | 27 | 31 | 58 | Synthetic | ||
| 59Co | 27 | 32 | 59 | 100% | Stable | |
| 60Co | 27 | 33 | threescore | Synthetic | ||
| 61Co | 27 | 34 | 61 | Synthetic | ||
| 62Co | 27 | 35 | 62 | Synthetic | ||
| 63Co | 27 | 36 | 63 | Synthetic | ||
| 64Co | 27 | 37 | 64 | Synthetic | ||
| 65Co | 27 | 38 | 65 | Synthetic | ||
| 66Co | 27 | 39 | 66 | Synthetic | ||
| 67Co | 27 | 40 | 67 | Synthetic | ||
| 68Co | 27 | 41 | 68 | Synthetic | ||
| 69Co | 27 | 42 | 69 | Synthetic | ||
| 70Co | 27 | 43 | lxx | Synthetic | ||
| 71Co | 27 | 44 | 71 | Synthetic | ||
| 72Co | 27 | 45 | 72 | Synthetic | ||
| 73Co | 27 | 46 | 73 | Constructed | ||
| 74Co | 27 | 47 | 74 | Constructed | ||
| 75Co | 27 | 48 | 75 | Constructed |
Co On The Periodic Table,
Source: https://www.schoolmykids.com/learn/periodic-table/co-cobalt
Posted by: curranyoughthears.blogspot.com


0 Response to "Co On The Periodic Table"
Post a Comment