What Is A Carbohydrate Monomer
ii.four: Carbohydrates
- Page ID
- 31764
Learning Objectives
- Requite examples of monosaccharides and polysaccharides
- Draw the part of monosaccharides and polysaccharides inside a prison cell
The about abundant biomolecules on earth are carbohydrates. From a chemic viewpoint, carbohydrates are primarily a combination of carbon and water, and many of them have the empirical formula (CHtwoO)n, where n is the number of repeated units. This view represents these molecules just as "hydrated" carbon atom chains in which water molecules adhere to each carbon atom, leading to the term "carbohydrates." Although all carbohydrates incorporate carbon, hydrogen, and oxygen, there are some that besides incorporate nitrogen, phosphorus, and/or sulfur. Carbohydrates have myriad different functions. They are abundant in terrestrial ecosystems, many forms of which we employ equally food sources. These molecules are also vital parts of macromolecular structures that store and transmit genetic information (i.e., Deoxyribonucleic acid and RNA). They are the basis of biological polymers that impart strength to various structural components of organisms (east.g., cellulose and chitin), and they are the primary source of energy storage in the form of starch and glycogen.
Monosaccharides: The Sweet Ones
In biochemistry, carbohydrates are frequently called saccharides, from the Greek sakcharon, meaning saccharide, although not all the saccharides are sugariness. The simplest carbohydrates are called monosaccharides, or simple sugars. They are the building blocks (monomers) for the synthesis of polymers or circuitous carbohydrates, equally will be discussed further in this department. Monosaccharides are classified based on the number of carbons in the molecule. General categories are identified using a prefix that indicates the number of carbons and the suffix –ose, which indicates a sugar; for case, triose (three carbons), tetrose (four carbons), pentose (v carbons), and hexose (half dozen carbons) (Effigy \(\PageIndex{1}\)). The hexose D-glucose is the most abundant monosaccharide in nature. Other very common and abundant hexose monosaccharides are galactose, used to make the disaccharide milk carbohydrate lactose, and the fruit sugar fructose.
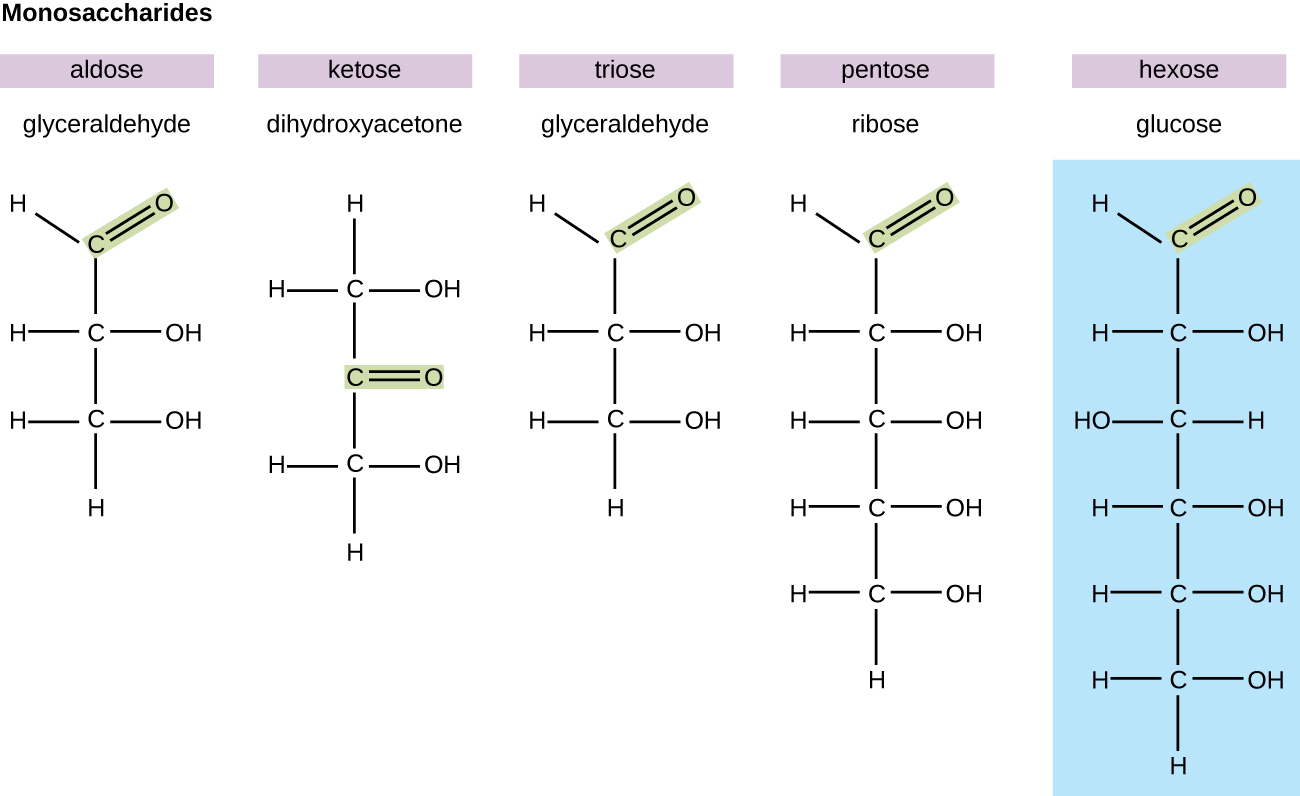
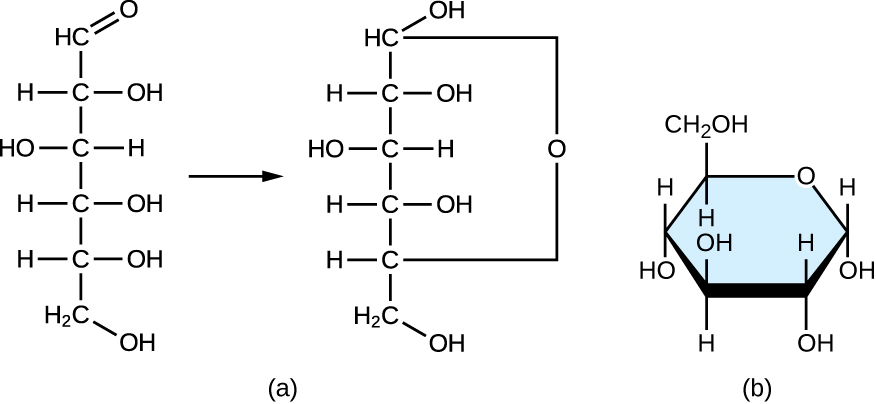
Monosaccharides of four or more carbon atoms are typically more stable when they prefer cyclic, or ring, structures. These ring structures result from a chemical reaction betwixt functional groups on contrary ends of the sugar's flexible carbon chain, namely the carbonyl group and a relatively distant hydroxyl group. Glucose, for example, forms a half dozen-membered band (Effigy \(\PageIndex{ii}\)).
Practice \(\PageIndex{1}\)
Why do monosaccharides class ring structures?
Disaccharides
Two monosaccharide molecules may chemically bond to form a disaccharide. The proper noun given to the covalent bail between the two monosaccharides is a glycosidic bond. Glycosidic bonds form betwixt hydroxyl groups of the two saccharide molecules, an example of the dehydration synthesis described in the previous section of this affiliate:
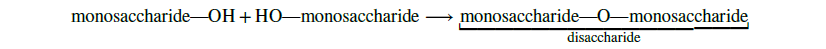
Common disaccharides are the grain carbohydrate maltose, fabricated of two glucose molecules; the milk sugar lactose, made of a galactose and a glucose molecule; and the tabular array sugar sucrose, fabricated of a glucose and a fructose molecule (Figure \(\PageIndex{3}\)).
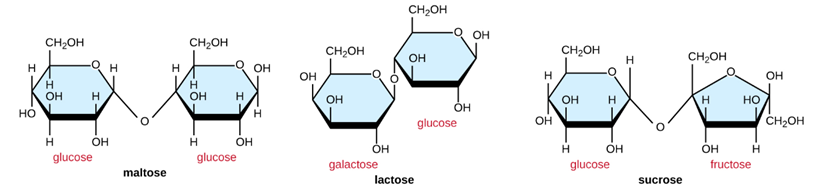
Effigy \(\PageIndex{3}\): Common disaccharides include maltose, lactose, and sucrose.
Polysaccharides
Polysaccharides, too called glycans, are large polymers composed of hundreds of monosaccharide monomers. Unlike mono- and disaccharides, polysaccharides are not sugariness and, in full general, they are not soluble in water. Like disaccharides, the monomeric units of polysaccharides are linked together by glycosidic bonds.
Polysaccharides are very diverse in their construction. Three of the most biologically important polysaccharides—starch, glycogen, and cellulose—are all composed of repetitive glucose units, although they differ in their construction (Figure \(\PageIndex{4}\)). Cellulose consists of a linear chain of glucose molecules and is a common structural component of cell walls in plants and other organisms. Glycogen and starch are branched polymers; glycogen is the primary energy-storage molecule in animals and bacteria, whereas plants primarily store energy in starch. The orientation of the glycosidic linkages in these iii polymers is different besides (Figure \(\PageIndex{5}\)) and, as a event, linear and branched macromolecules accept unlike properties.
Modified glucose molecules tin can be fundamental components of other structural polysaccharides. Examples of these types of structural polysaccharides are N-acetyl glucosamine (NAG) and North-acetyl muramic acid (NAM) establish in bacterial cell wall peptidoglycan. Polymers of NAG form chitin, which is found in fungal cell walls and in the exoskeleton of insects (Figure \(\PageIndex{v}\).
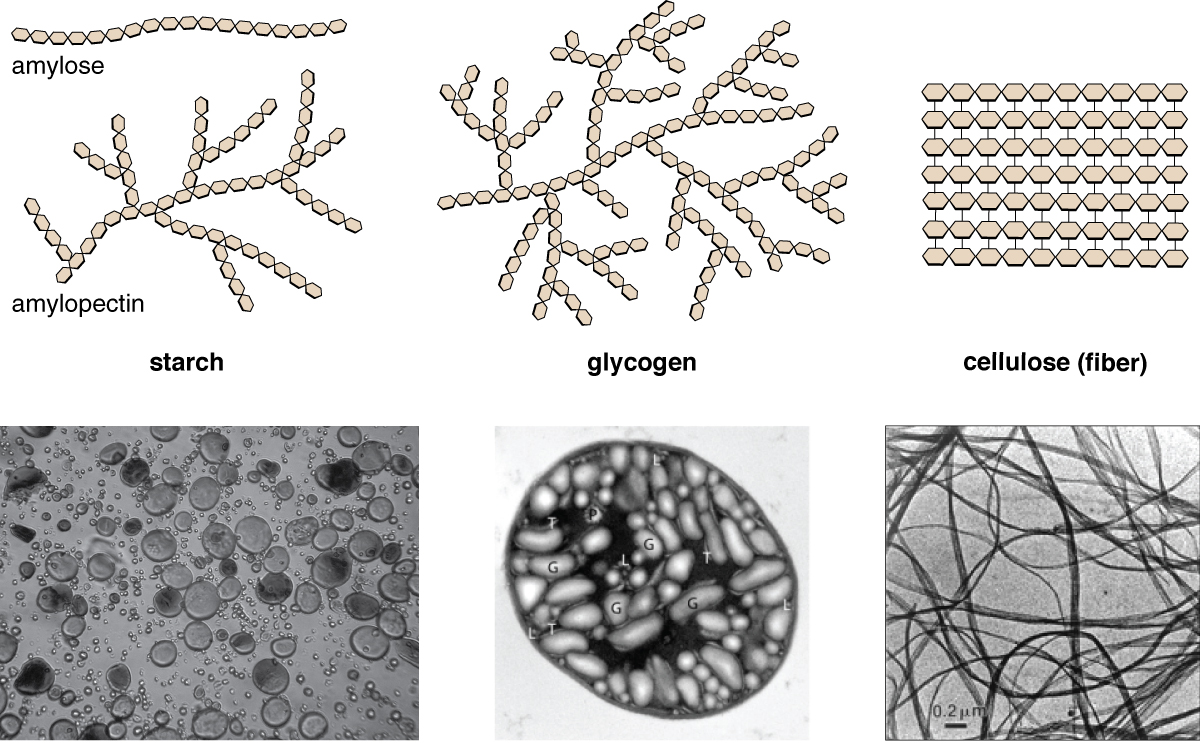
Figure \(\PageIndex{5}\): The linkages between the glucose molecules in starches or glycogen and structural carbohydrates like cellulose or chitin make a large departure in the stability of the molecule.
Practice \(\PageIndex{2}\)
What are the about biologically important polysaccharides and why are they important?
Key Concepts and Summary
- Carbohydrates, the most abundant biomolecules on world, are widely used past organisms for structural and energy-storage purposes.
- Carbohydrates include individual carbohydrate molecules (monosaccharides) besides as two or more molecules chemically linked past glycosidic bonds. Monosaccharides are classified based on the number of carbons the molecule equally trioses (3 C), tetroses (4 C), pentoses (five C), and hexoses (6 C). They are the building blocks for the synthesis of polymers or complex carbohydrates.
- Disaccharides such as sucrose, lactose, and maltose are molecules composed of ii monosaccharides linked together by a glycosidic bond.
- Polysaccharides, or glycans, are polymers composed of hundreds of monosaccharide monomers linked together by glycosidic bonds. The energy-storage polymers starch and glycogen are examples of polysaccharides and are all composed of branched chains of glucose molecules.
- The polysaccharide cellulose is a common structural component of the cell walls of organisms. Other structural polysaccharides, such as N-acetyl glucosamine (NAG) and N-acetyl muramic acid (NAM), contain modified glucose molecules and are used in the construction of peptidoglycan or chitin.
Contributors and Attributions
-
Nina Parker, (Shenandoah University), Mark Schneegurt (Wichita State Academy), Anh-Hue Thi Tu (Georgia Southwestern State Academy), Philip Lister (Central New United mexican states Community College), and Brian M. Forster (Saint Joseph's Academy) with many contributing authors. Original content via Openstax (CC Past iv.0; Admission for gratis at https://openstax.org/books/microbiology/pages/one-introduction)
What Is A Carbohydrate Monomer,
Source: https://bio.libretexts.org/Courses/Manchester_Community_College_%28MCC%29/Remix_of_Openstax:Microbiology_by_Parker_Schneegurt_et_al/02:_Chemistry_and_Biochemistry/2.05:_Carbohydrates
Posted by: curranyoughthears.blogspot.com


0 Response to "What Is A Carbohydrate Monomer"
Post a Comment